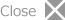Laboratory of bioinorganic chemistry
3 | Molecular mechanism of metalloproteins and metalloenzymes
Metalloproteins and metalloenzymes are a class of biologically important macromolecules, which have various functions such as oxygen transport, electron transfer, oxidation, and oxygenation. Metalloproteins and metalloenzymes have metal ions in their active sites. Although we use only about ten kinds of metal ions, such as iron, copper, zinc, and so on, the functions of metalloproteins and metalloenzymes are diverse. These diverse functions have been thought to depend on the ligands from amino acid, coordination structures, and protein structures in immediate vicinity of metal ions. We are studying the relationship between the electronic structures of the metal active sites and reactivity of metalloproteins.
Fig. 1 Heme in hemoglobin is degraded by heme oxygenase to a-biliverdin, which is finally oxidized to a-bilirubin. This is important biological process to produce bile pigment and to recycle iron ion, and is catalyzed by heme oxygenase. This picture shows color change in the heme degradation process.
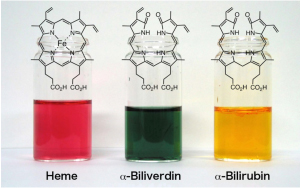
Fig. 2 We studied molecular mechanism of heme oxygenase (left). We found essential amino acid residues to have heme degradation activity, aspartic aicd-140 and arginine-183. These amino acids work as the knife and hand for regioselective heme oxidation (middle). Heme oxygenase works as a excellent chief.
4 | Photophysical properties of designed metalloproteins
Photoinduced electron-transfer (ET) and energy-transfer (ENT) reactions within a metalloprotein or metalloenzyme matrix to transport the electron initiated by the light such as photosynthetic systems have received considerable attention in the fields of both chemistry and biology and play important roles. In order to elucidate the complicated mechanisms of photoinduced ET reactions within metalloprotein matrices, we carried out various mechanistic and design approaches based on photochemistry of metal complexes. To date, much effort on the photoinduced ET reactions using hemoprotein, containing a Heme as a cofactor (Fig. 1),and DNA by time-resolved laser flash photolysis have been undertaken. We recently construct an enzyme-ligand complex by using a photosensitizer and a wire molecule (Fig. 2) . By using preferential protein-ligand interactions in an aqueous solution, we study the photophysical property of an artificial metalloprotein complex and the detailed ET mechanisms are discussed.